View Product
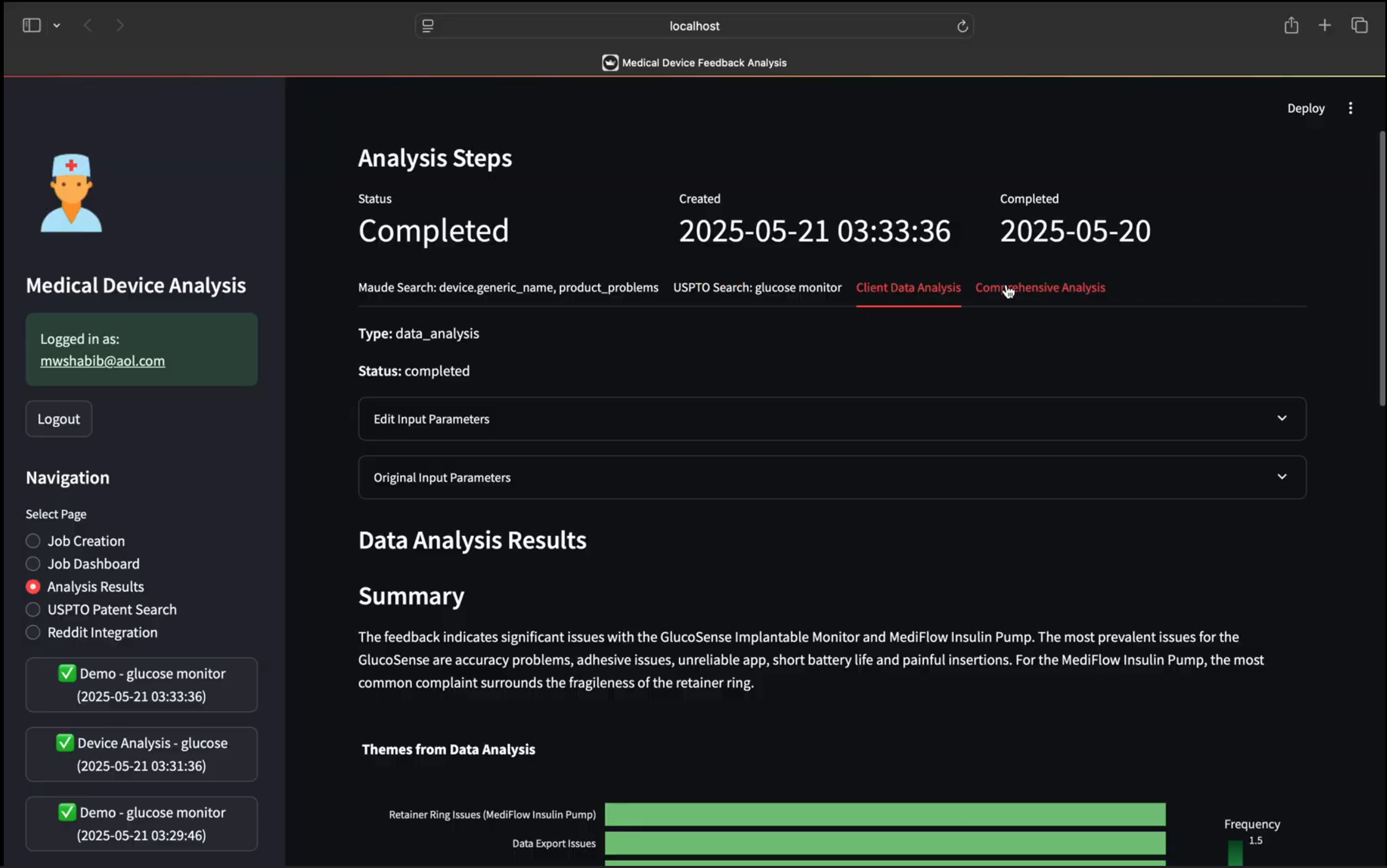
Spot critical device issues before they impact patients by revealing risk signals across regulatory and real-world data.
Automatically ingest and synthesize internal reports, FDA filings, social media, and patents into one intelligence platform.
Accelerate innovation by matching failure themes with proven solutions from across the industry and IP landscape.
Aggregate MAUDE reports, Reddit posts, patents, and internal feedback
Extract patterns and trends using AI
Generate design-ready insights with regulatory and technical context
Quickly detects recurring device issues from unstructured data like narrative MAUDE reports and Reddit posts.
Connects the dots between internal complaints, regulatory filings, and public sentiment to uncover hidden risk patterns and emerging concerns.
Identifies relevant prior art and published solutions so your team can move faster with confidence and regulatory alignment.
Export insights into structured documentation with traceable references for compliance and audits.



PIP helps innovators across the medical device ecosystem transform feedback into smarter design decisions.
Speed up regulatory learning and avoid known pitfalls using insights from real-world failures.
Supplement your own internal data with FDA databases and online patient feedback to continuously refine device design.
Access structured design data to support clinical studies, funding applications, IP strategy, and early validation.
Spot patterns, ensure traceability, and streamline evidence-based documentation for premarket planning.
See how innovators in the medical device space could use PIP to uncover insights from unstructured data like MAUDE reports and user feedback.
Click to expand
"Are our cardiac monitor battery issues an isolated problem or industry-wide?"
"Why are our spinal cord stimulator leads failing after implantation?"
"What user errors are causing our home dialysis machine alarms?"
PIP (Product Improvement Platform) is an AI-powered tool that transforms fragmented feedback—like adverse events, user complaints, and regulatory data—into actionable design insights for medical devices. It helps teams uncover why device failures happen and how to prevent them before market launch, reducing R&D uncertainty.
Unlike general-purpose GenAI platforms, PIP is purpose-built for medical device design and lifecycle decision-making. It integrates semi-structured regulatory data, unstructured real-world user feedback, patent filings, and client-specific inputs to identify device-relevant risks, failure modes, and design opportunities. Rather than simply answering questions, PIP synthesizes and contextualizes signals across traceable, authoritative data sources to generate actionable insights tailored to a specific device, indication, and market—while reducing the risk of hallucinations and unsupported outputs.
Unlike existing post-market surveillance tools or quality management systems that focus on documenting, tracking, or reporting issues within a single system, PIP operates upstream. PIP is not just a documentation platform—it actively searches across and synthesizes multiple external and internal data sources, including adverse event reports, real-world user feedback, patents, and regulatory guidance, within a single unified platform. By connecting signals across datasets rather than siloed databases, PIP helps R&D teams understand what to build and why—revealing unmet user needs, known failure modes, and competitive design patterns before formal development, quality, and compliance workflows begin.
No. Clients' uploaded data are not shared with other PIP clients. Privacy and security are core design principles.